 |
|
 |
Reverse osmosis, also known as hyperfiltration, is the finest filtration known. This process will allow the removal of particles as small as dissolved individual ions from a solution. Reverse osmosis is used to purify water and remove ions and dissolved organic molecules. It can be used to purify fluids such as ethanol and glycol, which will pass through the reverse osmosis membrane, while rejecting other ions and contaminants from passing. The most common use for reverse osmosis is in purifying water. It is used to produce water that meets the most demanding specifications that are currently in place. |
 |
|
|
If two aqueous solutions of different salinity are separated by a semi-permeable membrane, osmosis will cause water to pass through the membrane in the direction of the more concentrated solution, therefore diluting it. By applying sufficient pressure to the more concentrated liquid, the direction of osmosis can be reversed. In this way, we can mechanically reverse the flow and separate the concentrated solution into its constituents: the water and the dissolved solids. One part is called the permeate, or filtrate, and the other is the reject stream, or concentrate. |
Reverse osmosis uses a membrane that is semi-permeable, allowing the fluid that is being purified to pass through it, while rejecting the contaminants that remain. Most reverse osmosis technology uses a process known as crossflow to allow the membrane to continually clean itself. As some of the fluid passes through the membrane the rest continues downstream, sweeping the rejected species away Audemars Piguet Royal Oak replica watches
from the membrane, in a concentrated brine reject water. The process of reverse osmosis requires a driving force to push the fluid through the membrane, and the most common force is pressure from a pump. The higher the pressure, the larger the driving force. As the concentration of the fluid being rejected increases, the driving force required to continue concentrating the fluid increases.
|
Reverse osmosis is capable of rejecting bacteria, salts, sugars, proteins, particles, dyes, and other constituents that have a molecular weight of greater than 150-250 daltons. The separation of ions with reverse osmosis is aided by charged particles. This means that dissolved ions that carry a charge, such as salts, are more likely to be rejected by the membrane than those that are not charged, such as organics. The larger the charge and the larger the particle, the more likely it will be rejected. |
| |
Reverse Osmosis Principles |
|
|
 |
|
| |
 |
|

|
| To understand reverse osmosis we must first understand osmosis. During natural osmosis, water flows from a less concentrated solution through a semipermeable membrane to a more concentrated saline solution until concentrations on both sides of the membrane are equal (see figure 2).
|
 |
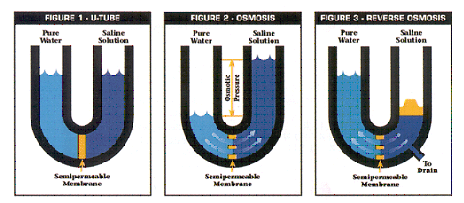
|
|
|
|
Reverse osmosis requires external pressure to reverse natural osmotic flow. As pressure is applied to the saline solution, water flows through the semipermeable membrane (see figure 3). |
|
| |
 |
A reverse osmosis membrane has a thin microporous surface that rejects impurities, but allows water to pass through. The membrane rejects bacteria, virus, pyrogens, and 85%-95% of inorganic solids. Polyvalent ions are rejected easier than monovalent ions. Organic solids with a molecular weight greater than 300 are rejected by the membrane, but dissolved gases pass through. Reverse osmosis is a percent rejection technology. The purity of the product water depends on the purity of the inlet water. The purity of reverse osmosis product water is much higher than the purity of the feedwater (see figure 4) Hublot King Power Replica
. |
I |
|
| |
|

|
A large percentage (50-90%) of the feedwater does not pass through the membrane but flows across the membrane surface, constantly cleaning it and carrying the inorganic and organic solids to drain. This water is called "reject." |
| |
|
 |
| Feedwater factors affecting membrane performance and life include the following: |
| |
| Pressure :
Feedwater pressure affects both the quantity and the purity of reverse osmosis product water. Lower feedwater pressure causes lower product flow rate and lower product purity. |
| pH :
Feedwater pH range is important. It is recommended that you use wider pH range membranes when feedwater is basic, acidic or unstable. |
Langlier Saturation Index (LSI) :
The LSI indicates the tendency for scale to form on a membrane surface. It requires feedwater testing for temperature, total inorganic solids, calcium hardness alkalinity, and pH. If the LSI index calculation is positive, it is recommended that you install a water softener prior to the reverse osmosis system. |
| Free Chlorine and Bacteria :
Cellulose acetate membranes require constant free chlorine to prevent bacterial growth and membrane damage. In contrast, polyamide and thin film membranes are damaged by free chlorine. Activated carbon is used to remove free chlorine when polyamide and thin film membranes are chosen. |
Temperature :
Membrane performance is based on feedwater temperature of 25°C. For every 1°C below 25°C product water quantity is reduced by 3%. When feedwater temperature is regularly below 25°C, it is recommended that hot and cold water are mixed to increase the temperature to 25°C. Feedwater which is greater than 35° will damage most membranes. |
Silt Density Index :
The SDI is a measurement of submicron particles and their tendency to block membranes. Flowing water at specific pressure is filtered through a membrane disc and collected for a fixed period of time. The speed of water flow and total volume collected determines the index value. |
Turbidity :
Turbidity is a measurement of suspended submicron particles that obscure light rays. |
| |
|
 |
Membranes are thin films of porous material, which can be used for a number of chemical separations. Although many membranes are made from polymer films, membranes can be formed from ceramics, carbon fiber, and porous metal substrates. The pores can range from atomic dimensions (< 10 angstroms) to 100+ microns. |
| |
|
|
| |
The small pores of the membranes (.0001micron) can serve as a physical barrier, preventing passage of certain materials such as salt, bacteria and viruses while allowing the free passage of water and air. The desalination of water using reverse osmosis is a well known use of membranes as a filter.
|
Recently, recovery of water from sewage and recovery of whey protein from waste streams during cheese making have been carried out with ultra filtration and micro filtration membranes which require much less pressure than reverse osmosis. While pressure is to be used to drive filtration, electrical current, osmotic pressure, and temperature can also be used to preferentially allow one component in a mixture to pass freely through the membrane while retaining the rest. |
The membrane structure and chemistry can also serve to carry out other separations.
Membranes provide a high surface area material where chemical reactions or diffusion can take place. For example, bundles of hollow fiber membranes (membranes in a thin tubular form) are used in dialysis to purify the blood by removing certain toxins. Membranes can also be used to carry out solvent extraction and catalysis while also serving to separate the reactants. |
Hydrophobic membranes can be used to prevent passage of liquid water but allow vapor to pass (like Goretex). This property has been exploited in membrane distillation where brackish water is heated using solar power and the pure water vapor passes through the membrane and condensed to produce very high quality water. This uses less energy than boiling and utilizes bountiful but low value energy in remote areas |
| ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,, |
|
|
|
|
